Process Of Third Party Contract Manufacturing
Third party manufacturing business in the pharmaceutical industry is growing at a very fast pace globally and is expected to increase at a fast rate over the next few years.
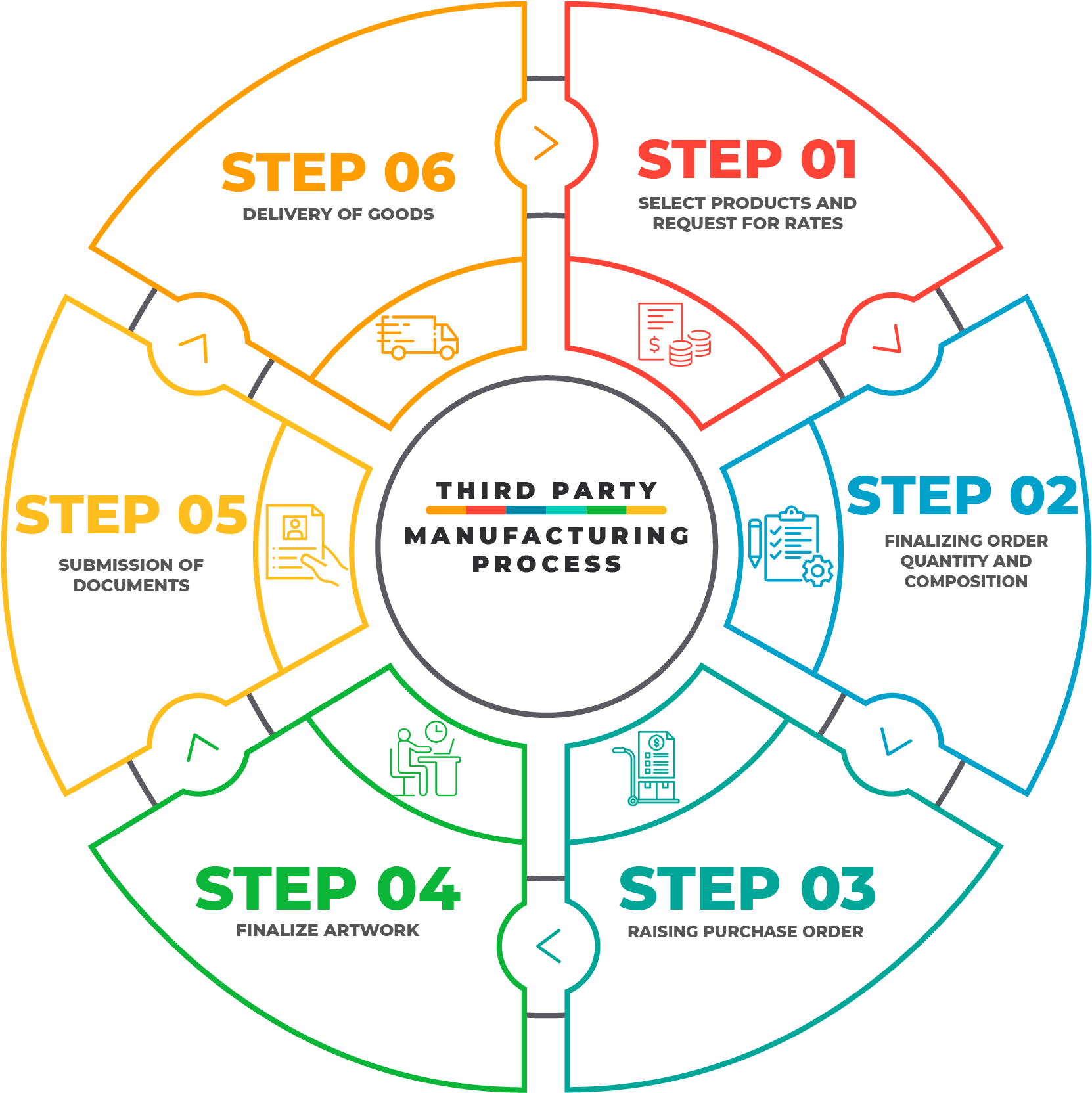
Every manufacturing business company is using quite similar technologies and equipments. The third party manufacturing process consists of multiple steps, and for each step there are process parameters with some control limits.
At Amster Labs Limited, our process involves:
-
Selecting desired products and request for rates
-
Shortlisting potential manufacturing and selecting desired products.
-
Call / Email requesting for their quotation which will include: -
-
Product Cost
-
Product Manufacturing Expenses
-
Min. Quantity of Delivery Schedule
-
-
-
Finalizing Order quantity and composition.
-
Raising Purchase order : Purchase order is raised and confirmation is made. Partial advance may be deposited to initiate the process
-
Finalize Artwork : Key points while finalizing artwork:
-
Check Brand Name on carton and foil.
-
Check packaging details, compositions, manufacturing details.
-
Check design and color combination
-
Check Marketed by Company Name, logo & Address on carton & Foil.
-
-
Submission of Documents:
-
Company Profile
-
Directors' Documents (Aadhaar Card & PAN Card)
-
Copy of Resolution for Authorized Signatory
-
Drug Licenses
-
Sales Tax / TIN Registration Certificates
-
Agreement of Manufacturing
-
Certificate of Non-Resemblance
-
-
Delivery of Goods: - Dispatch of goods through transporter after submission of documents and clearance of accounts.
Documents Required for 3rd Party Manufacturing
Profile:
(Brief Profile with Copy of PAN Card of the company and a Copy of Memorandum & Articles of Association in case of Pvt. Ltd or limited Company. Partnership deed/ Affidavit for proprietary in case of Partnership firm or Proprietorship firm.)
Name, Address & Telephones with copy of Aadhar Card and Pan Cards.:
(Of all Directors, Partners or Proprietor both official and residential.)
Copy of Resolution for Authorized Signatory to Deal
(For limited, private limited and partnership companies)
Drug Licenses:
(Attested copy of Drugs Licenses to be provided)
GST Registration Certificates:
(Attested copy of Sales tax Registration Certificate to be provided)
Agreement for Manufacturing:
(Specimen copy will be provided)
Certificate for Non-Resemblance:
(Specimen copy will be provided)

